PRODUCT
Business information
1. Pipeline
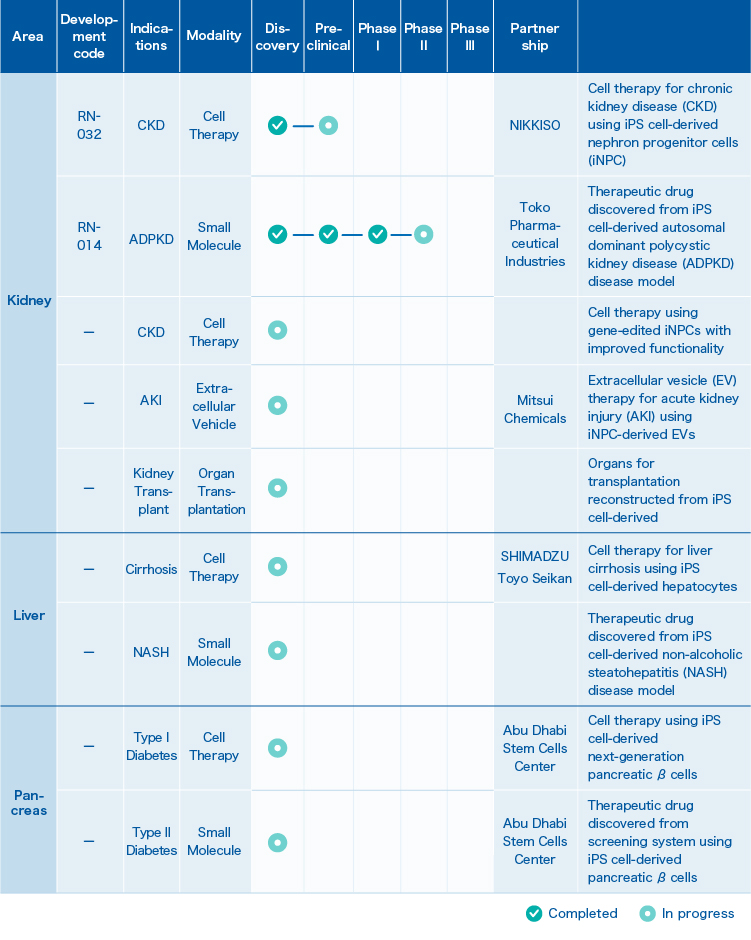
2. RN-032
Cell therapy for chronic kidney disease (CKD) using iPS cell-derived nephron progenitor cells (iNPC)
We have a very unique and realistic approach to R&D activities aimed at helping patients suffering from renal failure.
The basic role of the kidneys is to filter blood and eliminate unwanted water and waste products as urine. Blood throughout the body flows into the kidneys through the renal arteries that branch off from the aorta. Blood passes from the renal arteries through the gradually narrowing arteries into the thinnest arterioles, and is transported to the places called the glomeruli.
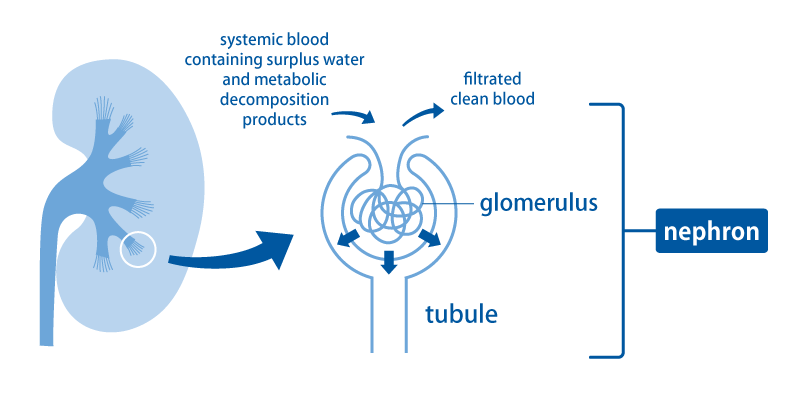
In the glomerulus, blood is filtered and becomes raw urine (the source of urine) that passes through the tubules. In the tubule, what is needed is reabsorbed and transported back to the blood. This small unit consisting of a glomerulus and a renal tubule is called a nephron. There are about 1 million nephrons in one kidney.
Kidney disease is a disease in which the function of the kidney is impaired by impairing the glomeruli and renal tubules of the kidney. Once the kidney function is lost, it rarely recovers and progresses to a condition called chronic renal failure.
There are general therapy, diet therapy, drug therapy, dialysis therapy, plasma exchange therapy, etc. in the treatment of kidney disease, and the treatment method is decided according to the medical condition at that time, but none of them is a fundamental treatment method, is the current situation.
Scared of kidney disease are various complications.
(1) Symptoms such as swelling, high blood pressure, and pulmonary edema appear when water and salt excretion ceases to function.
(2) Inability to excrete acid and electrolytes leads to acidosis, which accumulates acid in the body, and hyperkalemia and hyperphosphatemia.
(3) If waste products cannot be excreted, uremic toxemia may occur, causing vomiting and consciousness disorder.
(4) Anemia occurs due to the inability to produce hematopoietic hormone.
(5) Impaired activation of vitamin D results in hypocalcemia and deterioration of bone mass and quality.
When renal function declines to such a state, doctors recommend renal replacement therapy such as dialysis or kidney transplant. If you decline the doctor’s recommendation here and leave you without dialysis or kidney transplantation, the symptoms and complications associated with reduced renal function will worsen. Eventually, complications such as heart failure will occur and you will be at risk of life.
Dialysis therapy is a general term for removing waste products (urea toxins) and excess water accumulated in the body due to renal failure.
Currently, there are about 330,000 dialysis patients in Japan, of which about 7,000 patients have been on dialysis for more than 30 years. Many patients are living with dialysis, according to their lifestyle.
Kidney transplantation is a treatment that restores renal function by transplanting a new kidney to patients with end-stage renal failure. It is one of the alternative treatments for dialysis in patients with end-stage renal disease. According to the life and death of the donated kidney donor, it is roughly divided into a cadaver kidney transplant and a living kidney transplant.
Compared to other countries, Japan is not progressing due to various problems in kidney transplantation, especially in brain death. In addition to ethical problems, there are various causes such as problems with the medical system. Currently, the number of cases is currently around 1,700 annually. This is very small compared to more than 20,000 kidney transplants in the United States each year.
Under such circumstances, pharmaceutical companies are rushing to develop small molecule therapeutic agents, but since the kidney is a complex organ and renal failure is a disease caused by multiple factors, very few small molecule therapeutic agents with a specific mechanism of action have been effective so far.
Accordingly, regenerative medicine treatment is the most awaited in this disease area.
RegeNephro’s approach is very unique and realistic.
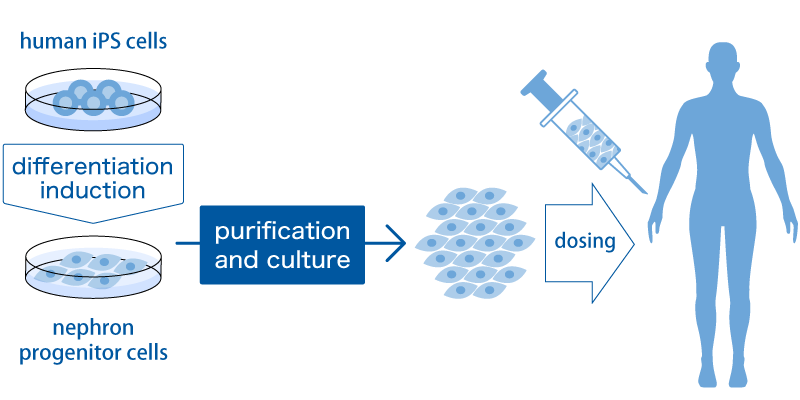
First, NPCs cells called nephron progenitor cells (NPCs) are created from iPS cells by inducing differentiation. NPCs are the cells that form the glomeruli and renal tubules, which are the filtration devices of the kidney described above. We believe that NPCs can be proliferated to a high level amount by the purification method and culture method we have developed so that they can be administered to humans. The kidneys are capsular, but NPCs are given between the kidney and the capsule using a special device. We assume that these NPCs will send various trophic factors to the kidneys and rejuvenate the broken kidneys. We believe that it is possible to bring about a paracrine effect directly and locally in the kidney by administering it under the capsule.
The efficacy of such treatment is now being confirmed in trials using small animals, and the development of the manufacturing process is progressing. In developing manufacturing processes, we are collaborating with partners who have a variety of specialized technologies. For example, we are jointly developing scale-up methods with NIKKISO CO., LTD.
By way of this new approach we at RegeNephro are desperately wishing to reduce the number of patients who shift to dialysis as much as possible.
3. RN-014
Development of a new therapeutic drug for autosomal dominant polycystic kidney disease (ADPKD)
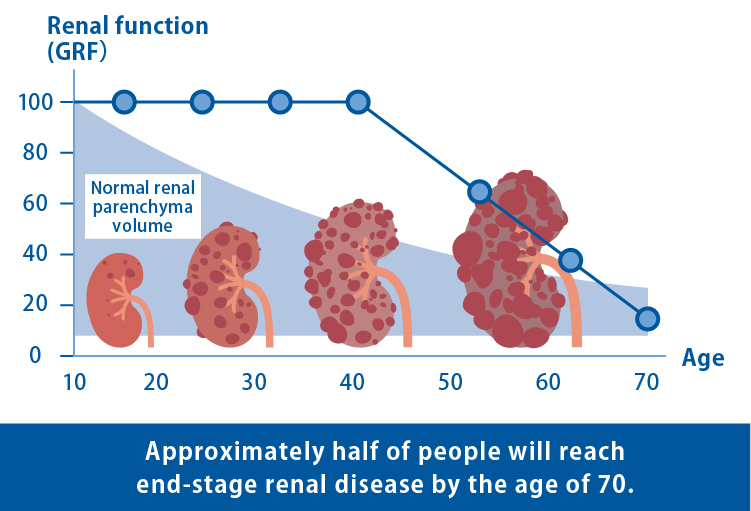
ADPKD is an intractable hereditary disease characterized by the gradual development and enlargement of numerous cysts (fluid-filled sacs) in the kidneys. The progression of kidney dysfunction often results in end-stage renal failure, requiring artificial dialysis or kidney transplantation in approximately half of patients by the age of 70. Although the drug tolvaptan has been approved to suppress the growth of cysts, there is currently no cure for ADPKD.
Numerous basic studies on ADPKD have been conducted using mice and rats. However, due to genetic differences in the biological species, therapeutic candidates that prove effective in mice and rats often fail to yield similar results in humans. Therefore, it was necessary to develop an effective disease model using human cells and explore novel therapeutic agents.
The research group of our Chief Scientific Advisor successfully generated a disease model replicating the symptoms of ADPKD by generating and differentiating iPS cells with mutations in the PKD1 gene, the causal gene for ADPKD, and discovered a novel candidate for the treatment of ADPKD (As announced on December 1, 2023). We initiated a clinical trial for this therapeutic candidate, a retinoic acid receptor (RAR) agonist in December 2023.
The RAR agonist identified as a therapeutic candidate is a drug that has been approved and used clinically as the treatment for relapsed and refractory acute promyelocytic leukemia (APL). Therefore, it is possible to start the clinical trials from the phase IIa (1), and the target population will be the ADPKD patients with a relatively high risk of disease progression who either cannot or do not wish to be treated with tolvaptan. Subject recruitment for this study will occur in phases to ensure the safety of the patients and will initiate in Japan once safety and tolerability are assured.
Please check the following website for details on clinical trials for RN-014.
jRCT (Japan Registry of Clinical Trials)
We have already received many messages of expectation from patients regarding the clinical trial. To deliver new therapeutics to patients as soon as possible and contribute to improving their quality of life, we will continue to thrive as a team.
Note (1) Phase IIa
A clinical trial that evaluates the efficacy and safety of a therapeutic candidate in a limited number of patients for whom it is expected to be effective, while also gathering information on the appropriateness of its usage and dosage.
